
Xenon samples are periodically extracted from the distillation column to evaluate the purity.
It is necessary to verify that one has reached the required purity levels. The extracted sample is sent to an external laboratory for the analysis. In particular, Krypton and Radon, which are the main radioactive contaminants in Xenon, can spoil the measurements carried out by the experiment.
In the XENON1T experiment xenon is periodically sampled. A sample tube which contains, approximately, a cubic centimeter of XENON, is sent to a specialized laboratory of the University of Heidelberg, in Germany, to be analyzed. The distillation process does not completely eliminate krypton, while it reduces its amount by about a factor of 500 thousand: from a few ppm (part per million, i.e. one krypton atom every million xenon atoms) to less than one ppt (part per trillion, that is one krypton atom every thousand billion xenon atoms). It is essential to keep the krypton below this concentration so that possible spurious events from krypton decays do not interfere with the interaction of dark matter particles with xenon (see DArk Matter). These very small amounts of krypton can be identified thanks to the use of two analytical techniques: gas chromatography, which allows to separate the krypton from the xenon, and mass spectroscopy, with which the krypton present in the sample is measured.
The gas chromatography is a method that exploits the different chemical affinity (an intrinsic property of the components that indicates the tendency to combine with other chemical elements) of a mixture with a transport gas (which is inert and is called "mobile phase") and with a liquid or solid substance sticking to a support (called "stationary phase"). The mixture is pumped with the mobile phase inside a long and narrow column whose walls are covered with a thin stationary phase layer. During the passage inside the column the different components of the mixture get combined to a greater or lesser extent to the stationary phase, based on their chemical affinity. The substances with a greater affinity to the stationary phase move slower in the column due to greater interaction with it, while the less similar ones are transported faster. At the end of the column there is a detector that generates a signal at the arrival of each of the components of the mixture. The final result is a chromatogram, i.e. a graph in which signal pulses are reported according to the arrival time (Fig. 1). From the position of the pulses along the time axis we obtain information on the type of substances, while the height provides an indication of their abundance in the mixture. The chromatograph used in the Heidelberg laboratories makes use of multiple columns. The first of these is to purify the carrier gas, in this case helium, from krypton, which must reach negligible levels in order not to contaminate the xenon. At this point, the xenon sample is injected into the instrument and, carried by the helium, enters into a second column a few meters long and 6 millimeters in diameter. The column is coated with a stationary solid state phase, called "adsorbent", with which xenon has a greater affinity with respect to krypton, under appropriate pressure and temperature conditions (atmospheric pressure and about -80 ° C). Then the krypton moves faster and enters into a third adsorbent column where it remains trapped, while the carrier gas flows out of the column. The krypton is released from the adsorbent material by heating the column and it can be then transferred to the mass spectrometer for quantification.
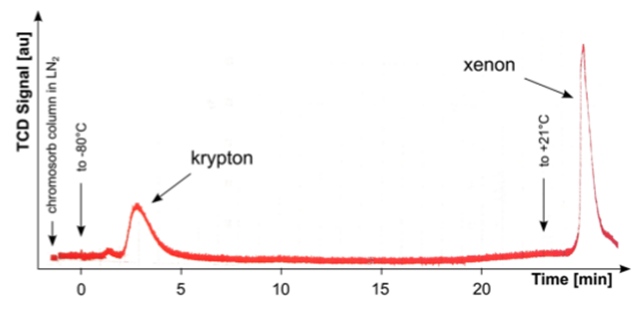
Fig . 1. Example of a chromatogram produced by the separation process of krypton and xenon. On the x axis is reported the time in minutes. The height and width of the peaks provide an indication of the amount of the substances. This chromatogram is produced by a thermal conductivity detector. A detector of this kind consists of two electrically heated filaments kept at constant temperature; in the volume where one of the two filaments is hosted the pure transport gas flows, while in the other one the gas leaving the column flows. The latter contains xenon and krypton, the passage of which removes some amount of heat from the filament, resulting in a change in temperature between this filament and the other one. The temperature variation is translated into a change in electrical resistance which is amplified and represents the detector output signal (credits: Eur. Phys. J. C (2014) 74: 2746)
Once the krypton that was present in the xenon sample is extracted, we proceed with the mass spectrometer analysis, a tool used to identify and quantify substances. This analysis is based on the application of a magnetic field to a beam of accelerated ions, which become separated according to their mass-to-charge ratio. In the first part of the spectrometer the ionization of the krypton sample takes place thanks to an electron beam. The ions are therefore focused and accelerated by an electric field inside a vacuum tube, in which a magnet is installed. The magnetic field deflects the ion beams, separating them according to their mass-to-charge ratio. In fact, if a magnetic field perpendicular to the momentum is applied to the moving particles, they are forced on a circular trajectory, with a radius of circumference depending on the mass-to-charge ratio: in general, lighter ions will suffer a greater deviation than heavier ones. Changing the intensity of the magnetic field the ion beams are directed towards a detector, which emits a signal proportional to the number of ions of each type. The result is displayed in a graph, the mass spectrum, which shows the relative abundance of the ions and their mass-to-charge ratio. It is thus possible to trace the type and quantity of substances present in the sample.
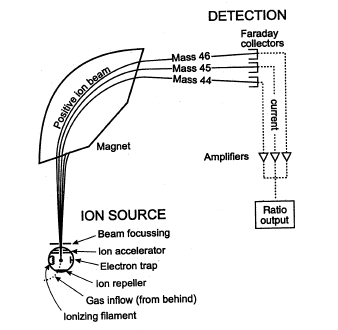
Fig. 2. Scheme of a mass spectrometer. The ion source is the sample of gas to be analyzed, introduced into the apparatus (Gas inflow) and bombarded by an electron beam. This is produced by a high temperature filament (Ionizing filament) and accelerated towards a positive electrode (Electron trap). The produced ions, which have a positive charge, are pushed by a plate which is also positive (Ion repeller) towards an accelerator (Ion accelerator), which is based on the electric repulsion and focuses them in a beam (Beam focusing) inside an empty tube. The positive ion beam is deflected by a magnet. Depending on the ratio between mass and charge of the ions, they undergo a greater or smaller deflection, and they are collected by a detector which amplifies the signal and sends the data to a computer (credits: Wikimedia Commons).

Discover the experiments and help the alien to get back home!
Play now