
Reproduces the nuclear reactions occurring in stars by hitting different types of targets with charged particle beams.
Almost all elements located in the Universe originate from the thermonuclear reactions occurring in the stars. These reactions produce energy and form the elements, from the lightest to the heaviest ones. Particles accelerators are used to understand these mechanisms reproducing fusion reactions in the laboratory.
Nuclear astrophysics aims to explain the origin of chemical elements and understand mechanisms producing energy in stars. At first impression it seems that these two aspects are not linked, but they are! Indeed, nuclear fusion reactions occurring in the stellar core are responsible both for the production of energy, which regulates the stellar evolution, and for the nucleosynthesis of almost all the elements of the periodic table. Without stars, the periodic table would be poor indeed. We would only have hydrogen, helium and lithium with their isotopes (that is, the same elements with an equal number of protons but different number of neutrons). These three elements are the lightest existing in nature, with one, two and three protons in their nucleus, respectively. They were formed in the first three minutes of the life of the universe during the process called Big Bang Nucleosynthesis. All the other elements, including most of those which make up the Earth, appeared when the stars began to “light up”, starting an evolution cycle still on going today. In particular, light elements started to merge into the core of the first stars, billions of light years away from us, gradually producing heavier and heavier elements. When this nuclear fuel became used up, stars collapsed and scattered the synthesized elements out into the universe through explosions (supernovae) (See solar neutrinos) or they expanded into red giant stars. This material was collected in clusters in interstellar space. Thanks to gravitational force, these globular clusters collapsed generating a second generation of stars. This cycle repeats itself, synthesizing all the existing elements. Nuclear astrophysics studies the nuclear reactions taking place in stars. In fission nuclear reaction energy is produced by breaking one nucleus. In contrast however, fusion reaction happens when two light nuclei merge thanks to very high kinetic energy given by high temperatures. This high energy overrides electromagnetic repulsion. This process releases a huge amount of energy! A star is a gigantic nuclear fusion reactor. For example, the Sun (quite a small star) burns about 600 million tons of hydrogen per second. About 99.3% is merged into Helium and the remaining 0.7% becomes energy, mainly emitted as gamma rays. This is only a small percentage, but the energy emitted is about 4 million times larger than the electric energy produced in the World in one year! The series of reactions described – which also generates a huge number of neutrinos (See solar neutrinos ) – is called the proton- proton (p-p) chain. Another series of reactions takes place in more massive stars and is called the Carbon-Nitrogen-Oxygen (CNO) cycle, because these elements are involved and allow 4 protons to melt into one Helium nucleus. Other fusion reactions that produce all the elements right up to iron (Z= 56) take place in massive stars (with mass 8 times larger than the Sun). Elements heavier than iron are synthesized in processes inside supernovae. The understanding of all these reactions helps to understand the evolution of the stars and the origin of the elements present in nature. For this, it is crucial to estimate the reaction rates that indicate the probability that each reaction may take place. In a nut shell, the main goal of experimental nuclear astrophysics is to reproduce stellar reactions and evaluate the reaction rate.
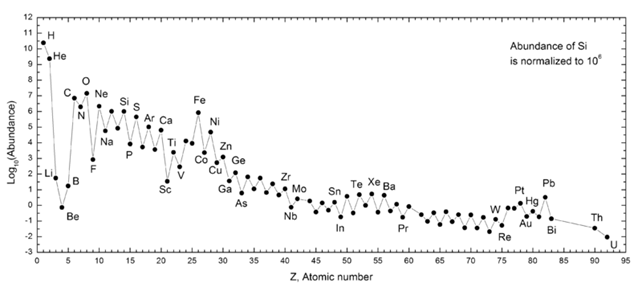
Fig 1. A graph showing abundance of elements in the solar system. On x axis, the Atomic number Z indicates the number of protons contained in the nucleus of the element. On y axis, the relative quantities of the elements expressed in powers of 10. For example oxygen (Z=8, indicated with O, about ten to the seventh) is 4 orders of magnitude more abundant than Fluorine (Z=9, indicated with F, about ten to the third). It is possible to notice that the higher the mass, the less abundant the element in our Solar System (Credits: Wikipedia Commons)
The reproducibility of a fusion reaction in a laboratory is not an easy task. These reactions have a low probability of occurring and thus observation is very rare. They are hidden among a lot of other processes, among which the environmental background coming from the radioactivity of the objects in the room and from the cosmic rays (See muon veto) For this reason, LUNA (Laboratory for Underground Nuclear Astrophysics) is installed in the LNGS, shielded by 1400 meters of rock (the Gran Sasso massif). LUNA was the first, and until 2017 the only, nuclear astrophysics experiment in an underground laboratory in the world. LUNA uses a particle accelerator (See accelerator), a machine that accelerates charged particles up to the speed of light confined in a beam particle by means of electromagnetic fields. LUNA is an electrostatic accelerator. This means that particles are accelerated by a constant electric field. The high voltage system is inside a cylindrical tank filled with an insulator gas avoiding sparks. LUNA can accelerate hydrogen or helium particles, obtaining a beam with a radius of few millimeters. These particles are extracted from a hydrogen gas through an ionization process: a very strong electric field removes one electron from the gas atoms. The beam travels through a 3 meter long beamline. To prevent degradation before the beam reaches its target, the beam is pumped in vacuum. Thanks to a magnet two different beamlines can be selected: one ends with a solid target and the other with a gaseous target. The beam impinges the target and nuclear reactions occur, producing gamma rays or charged particles. Different reaction products are detected by different particle detectors. Charged particles are detected by silicon detectors, based on a semiconductor that is insulator but becomes conductor when it is crossed by a particle. Photons (gamma rays) are detected by another type of semiconductor, a High Purity Germanium detector (HPGe) or scintillator detector. The latter are crystals that emit ultraviolet light when crossed by gamma rays that excite its molecules. All the detectors are shielded with lead or copper structures that further reduce the background radiation coming from the rock above the laboratory.
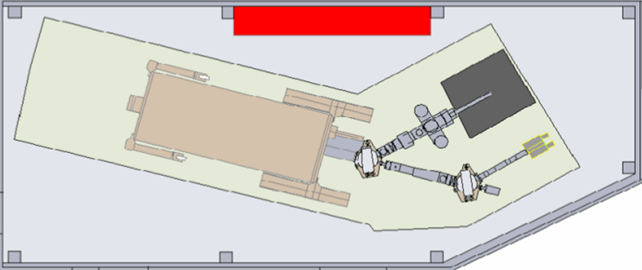
Fig 2: Diagram of the LUNA experiment. From left to right, one can see the 2.8 meter long tank (brown rectangle, a cylinder in reality) where the beam is generated and then directed towards one of the two beamlines (gaseous or solid target)
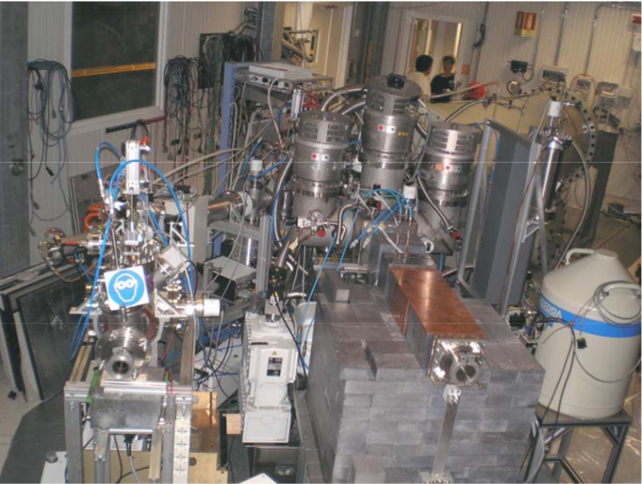
Fig3: The LUNA experiment apparatus: in the foreground the two targets (gaseous target on the right, shielded by lead; solid target on the left). In the background one can see the beamline.

Discover the experiments and help the alien to get back home!
Play now