Measures the radiation emitted by a material, such as lead, identifying which and how many radioactive elements are present inside.
The alpha spectrometer, as implied by its name, is able to detect alfa particle radiation emitted by radioisotopes that ‘’decay’’, meaning that they pass from an unstable condition to a more stable one, thereby emitting an alpha particle consisting of two neutrons and two protons. The spectrometer allows to find which radioactive element has produced emissions and to determine its abundance. Lead emits alpha radiations in variable intensity, based on its age.
Some elements, including all those heavier than lead, are radionuclides, that is, they undergo radioactive decays. In these processes, an initially unstable nucleus loses energy through the emission of one or more different particles. Depending on which particles are emitted, radioactive decays are generally called alpha, beta and gamma. In beta decay (and its variants, such as electron capture and inverse beta decay) a nucleus changes its nature and this can happen in two different ways. In the first there is the transformation of a neutron into a proton, accompanied by the emission of an antineutrino and an electron; in the second, instead, the proton transforms into a neutron, emitting this time a neutrino and a positron; antineutrino and positron are the antimatter particles corresponding to neutrino and electron.
Very often following the beta decay, the nucleus of the final atom is not "stable" and undergoes an "energetic rearrangement" which culminates in the emission of gamma rays, photons of very high energy, 10-100 times more energetic than X-rays normally used in radiology. In the alpha decays, the transformation involves the emission of a helium nucleus, a charged particle composed of two neutrons and two protons, also called an alpha particle. Unlike the other decays, the alpha particle stops in a few centimeters of air, even if it has energy greater than gamma rays. Each radioactive element leaves its particular "signature", linked to the type of radiation and its energy. Therefore, if this signature is found in a material, it can be concluded that the (radioactive) element is present. In some materials, the presence or absence of such a signature depends on the age of the material itself. Particularly ancient lead ( See Roman Lead) for example, loses all its initial radioactivity over time and is an excellent material for shielding experiments such as CUORE from environmental radiation.
The instrument specialized in identifying the radioactive elements that undergo alpha decay within a material is called alpha spectrometer. Alpha emissions have the characteristic of being absorbed very quickly, disappearing in a short time. In fact, due to their charge (2+, given by the presence of two protons) and large mass, the helium nuclei interact very much by means of the electromagnetic force with the matter they pass through, being absorbed by it very easily: alpha radiation can be absorbed by a simple sheet of paper or by the outer layer of human skin the thickness of a few cells. In order to detect these particles, alpha spectrometers usually use so-called semiconductor materials, for example silicon crystals. The properties of these crystals ensure that an area can be created inside them where the passage of even very weak radiation generates a measurable electric current flow. Let's see in more detail how.
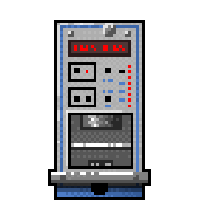
A semiconductor can be "doped", that is so to speak, soiled with impurities, in a way that inside there is a certain amount of electrons in excess (n-type semiconductor) or in defect (p-type semiconductor). In the first case the electrons carry negative charge, while in the second the "gaps" created by their absence act in all respects as positive charge carriers. By connecting two thin p and n layers, an area called the pn junction is created at the interface between the two surfaces, a portion of space that, with the application of an electrical voltage, can be made neutral and "impermeable" to the passage of charges from one part of the semiconductor to the other. When this occurs, an electric field is formed in this area which immediately expels any charge that is generated inside it. Under these conditions, when an external charged particle crosses this space, it interacts with the electrons of the material, ionizing them - extracting them from their atoms - creating inside the junction hundreds of thousands of electron-hole pairs that are immediately expelled, generating a current impulse measurable by the detector. The energy of the particle is obtained from the intensity of the impulse – the radiation that we want to identify – that generated it. This is the operating principle that uses an alpha spectrometer. On a practical level, in these instruments, a thin, suitably treated layer of sample of the material to be analyzed is placed near the detector, inside a volume where vacuum is created. Vacuum is essential since alpha radiation could be absorbed by the air before reaching the detector. The latter is coupled to a circuit that amplifies the electrical signal (since the energy deposited by alpha radiation is typically very small) and to a software that is able to process these signals, that is, to discriminate them over time (they can be very close) and to calculate their energy. As complex as it is, an alpha spectrometer has very small dimensions, roughly half of a microwave oven (Fig. 1).

Fig.1 An alpha spectrometer, composed of three independent modules (Image credits: Canberra Industries).
The final result of the analysis of a material is the spectrum of radiation emitted: a graph showing the measured energy on the horizontal axis and the number of particles that have deposited that specific energy on the vertical axis (Fig. 2). Since every radioactive element emits radiation of a certain energy, of which we know the value, from the spectrum analysis it is possible to understand which radioactive elements are present in the sample. For example, knowing that a given element emits radiation of energy X, to understand if it is present in the sample, it is enough to see if there is a peak in the spectrum at the X value. Furthermore, the higher the peak, the greater the quantity of the radioactive element present in the material. Alpha spectroscopy is a technique widely used to test the properties of materials, make geological or mineralogical analyses and monitor environmental radioactivity.
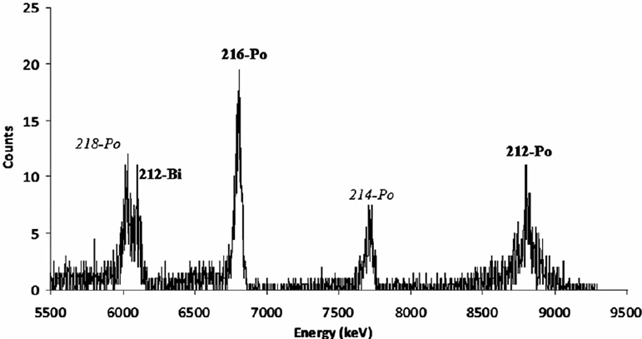
Fig.2 Example of an alpha spectrum. On the x-axis the values of the energy released and on the y-axis the number of radiations counted. The peaks correspond to radiation emitted by radioactive isotopes of polonium (Po) and bismuth (Bi) (Image credits: from Buompane et al. Radiation Protection Dosimetry, 2014, p. 1-4).
An alpha spectrometer isn't the only tool used to measure radiation. A well known and particularly simple one is the Geiger counter, which emits a particular sound similar to a "crack" every time it reveals a radiation. A Geiger counter is essentially made up of a tube filled with a gas to which an electric field is applied. When radiation enters the tube, it ionizes the gas electrons which are accelerated towards a positive electrode (anode) by exciting and ionizing other electrons in an avalanche effect. The avalanche is very short and creates an intense pulse of current which is revealed and results in a count. This instrument – much cheaper than an alpha spectrometer – is capable of detecting alpha, beta and gamma radiation, but limits itself to counting, without giving any information on the radiation energy it reveals (it does not produce any spectrum). Unlike the alpha spectrometer, the Geiger counter gives an indication of the amount of radiation emitted, but not of its nature and therefore does not allow us to trace which elements generated it.
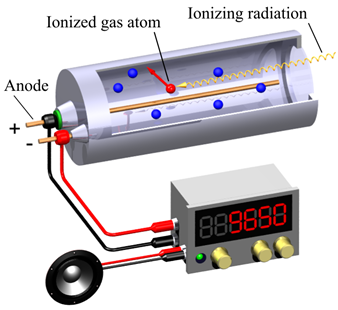
Fig.3 Graphical representation of a Geiger counter. The ionizing radiation enters the tube by ionizing the gas atoms (ionized gas atom), which accelerated by the electric field produce an electric impulse due to an avalanche effect. The impulse results in a count on the display of the instrument, and in a noise similar to a "crack" by means of a small amplifier (Image credits: Wikimedia Commons).

Discover the experiments and help the alien to get back home!
Play now