Material that absorbs energy from particles, which pass through it and re-emits it as light.
Many physics experiments use a scintillator, a material that emits light (photons) as particles pass through it. The emitted photons are detected by photomultipliers, which transform the light into a measurable electrical signal. Based on the signals features one can identify which particles have passed, for example a solar neutrino or a cosmic ray.
The scintillation process is the capability, of a wide range of materials, to emit light radiation (called luminescence) following its excitation. In fact, ionizing radiation (for example a charged particle or a photon) impinging a scintillator detector could release part of its energy into the scintillator material; such energy deposition is able to ionize or excite the molecules of the scintillator detector. After a very short time, the excited/ionized molecules come back spontaneously to the neutral condition with the prompt emission of light radiation. Usually this light is a visible radiation (fluorescence) or an ultraviolet radiation (phosphorescence). The light emitted, called scintillation light, is collected, often by using a guide light, into a photon detector as, for example, a photomultiplier (see photomultiplier). The photomultiplier converts the light signal into a current signal. Analysing the physical features of this current signal (for example shape, size, time duration) it is possible to get information about the interacting particle type and/or its initial energy. The ideal scintillation material should have the following characteristics: (i) it should convert the kinetic energy of charged particles into detectable light with a high scintillation efficiency; (ii) this conversion should be linear – the light yield should be proportional to deposited energy over as wide a range as possible; (iii) the medium should be transparent to the wavelength of its own emission for good light collection; (iv) the decay time of the induced luminescence should be short so that fast signal pulses can be generated; (v) the material should be of good optical quality and subject to manufacture in sizes large enough to be of interest as a practical detector; (vi) its index of refraction should be near that of glass (~ 1.5) to permit efficient coupling of the scintillation light to a photomultiplier tube or other light sensor. The scintillation material is of two types: organic and inorganic.
Organic scintillators are aromatic hydrocarbon compounds containing linked or condensed benzene- ring structures. As detectors, they have been used in the form of pure crystals and as mixtures of one or more compounds in liquid and solid solutions. Scintillation light in these compounds arises from transitions made by the free valence electrons of the molecules. These delocalized electrons are not associated with any particular atom in the molecule and occupy what are known as the π- molecular orbitals. A typical energy diagram for these orbitals is shown in Fig. 1. Ionization energy from penetrating radiation excites both the electron and vibrational levels as shown by the solid black arrow in Fig. 1 (S0 to S1 state). Excitations generally decay immediately to the lower energy levels without the emission of radiation (red solid line), a process which is known as internal degradation. From S1 state, there is generally a high probability of making a radiative decay to one of the vibrational states of the ground state S0 (green line) within a few nanoseconds. This is the normal process of fluorescence.
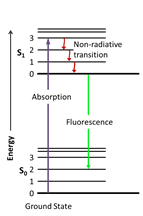
Fig.1 The fluorescence process. An electron is excited from its ground state to a higher level; it decays without emitting photons (non-radiative transitions) on an energy intermediate level, from which it can return to the ground state (green arrow) emitting the so-called fluorescence light.
Inorganic scintillators are mainly crystals of alkali halides containing a small activator impurity. By far the most commonly used material is NaI(Tl), where Thallium (Tl) is the impurity activator. Somewhat less common, but in active use is CsI (Tl), also with Tl as an impurity activator. Other crystals include CsF2, CsI(Tl), CsI(Na), KI (Tl) and LiI (Eu). Among the non-alkali materials are Bi4Ge3O12 (bismuth germinate or BGO), BaF2, ZnS(Ag), ZnO(Ga), CaW04 and CdW04 among many others. Often, also noble gases are used as scintillator inorganic materials. In general, inorganic scintillators are 2 - 3 orders of magnitude slower in light response than organic scintillators due to phosphorescence processes. The advantage of inorganic crystals lies in their higher density and higher atomic number. Among all the scintillators, they also have some of the highest light outputs, which results in better energy resolution. This makes them extremely suitable for the detection of gamma-rays and high-energy electrons and positrons. Whereas the scintillation mechanism in organic materials is molecular in nature, that in inorganic scintillators is clearly characteristic of the electronic band structure found in crystals. When a nuclear particle enters thecrystal it can ionize the crystal by exciting an electron from the valence band to the conduction band, creating a free electron and a free hole; or it can create an exciton. The resulting recombination or de-excitation can produce light emission, the scintillation.

Discover the experiments and help the alien to get back home!
Play now