
Water is an excellent shielding material against radiations, but must be very pure.
To reveal particles like solar neutrinos, there’s need of a large water shielding that surrounds the core of experimental apparatus to protects it from environmental radiation. Since water contains small amounts of radioactive contaminants it needs to be purified. The process includes several stages of filtration. At the and of the treatment the water is ultra-pure, millions of times less radioactive then at the beginning.
When carrying out any physics experiments aimed at observing very rare events it is of the utmost importance to reduce background environmental radiation, which could compromise the outcome. An easy way to achieve this goal is to surround the detector that is registering the events with a huge amount of water. This liquid shield reduces radiation and also helps identify any radiation that still manages to pass through and make its way to the detector. This way one can avoid counting those background events as part of the events one was effectively looking for (seemuon veto). Have we solved our problem? Not really, because water too is “radioactive”! Of course, its “radioactivity” is infinitesimal – our body is not affected in any way – but nevertheless, even this minute amount is a big problem when carrying out very sensitive experiments like Borexino, GERDA, Xenon1T at LNGS. To be precise, it is not the water in itself that emits radiation, but some naturally occurring elements that are present in very small amounts, e.g. potassium, radon, radium, uranium, and tritium. The water used as shielding material for experiments at LNGS must be purified from these contaminants. The purification process is very elaborate and is done in several steps as the required level of purity is extremely high. Here are the details.
The very first step is filtering the water to remove particulate matter (see filter) and material in suspension in the air such as fibres and dust, which can enter the water through contact with air.

Fig.1 A picture of a filter in step one, removal of particulates.
L’acqua Afterwards the filtered water undergoes reverse osmosis, a technique able to filter out undesirable substances present in the form of ions or bigger molecules. This technique makes use of special membranes (see reverse osmosis membrane) (Fig.2) that are permeable only to the solvent (water), but block a large part of the solutes (substances dissolved in the water) among which radioactive components. Basically, there are two solutions, one pure (diluted) and the other with substances dissolved in it (concentrated), separated from each other by the special membrane. Pressure is applied on the side of the concentrated solution. If we did not apply this external pressure, the natural osmotic process would go in the opposite direction, i.e. the solvent (water) would go from the pure diluted solution into the concentrated one. Applying external pressure on the concentrated solution the solvent (water) will pass from the concentrated solution to the pure diluted solution, i.e. the osmotic process is reversed, thus the contaminants are blocked by the special membrane increasing the purity of the diluted pure solution.
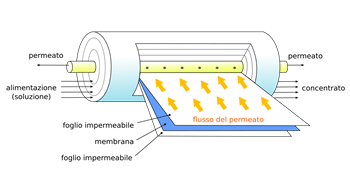

Fig.2: Membrane used in reverse osmosis and a schematic view of how it works. Water enters from the left, and due to pressure towards the center, passes through different layers of semi-permeable membranes as it moves towards the center, becoming purer and purer, before being finally collected in the central pipe. (Credits of larger image: Wikimedia Commons)
Reverse osmosis is followed by a degassing phase. The dissolved gasses are separated from the water by being filtered through another type of special membrane. After this step and yet another phase of reverse osmosis the water is then fed into the electro deionizing unit to purify it from any remaining ions. Inside the electro deionizer the water flows in a series of parallel channels: “purification” channels and “concentration” channels. In the purification channel the water passes through ion exchange resins (see ion exchange resins) (Fig. 3). These resins consist of small microbeads made of synthetic material that capture heavy ions from the water passing through them, releasing lighter ions. These ions are charged particles and therefore are affected by the electric field generated by two electrodes placed on the external walls of the electro deionizer: positively charged ions move towards the cathode (negatively charged electrode) and negatively charged ions towards the anode (positively charged electrode). The right and left walls of each purification channel are made of membranes that allow only positively or negatively charged ions to pass through to the adjacent concentration channels. With reference to figure 4, all the membranes on the right are permeable only to positive ions (cations) which are attracted to the right by the cathode, while all the membranes on the left are permeable to negative ions (anions) attracted to the left by the anode. Therefore, due to the electric field, the ions move through the membranes and are trapped in the concentration channels, while the purified water can flow away and then be collected.

Fig.3: A photograph of the ion exchange resin used in the purification process.

Discover the experiments and help the alien to get back home!
Play now