To purify Xenon one uses the phase changes between liquid and gas inside a column.
Xenon gas used to study dark matter has to be free of contaminants as much as possible, especially from radioactive gases, like Radon and Krypton. Evaporations and condensations are carried out to remove them. Xenon, Krypton and Radon have different properties in both phases, liquid or gaseous, that are function of pressure and temperature: by acting on these latter parameters, the gaseous Krypton and liquid Radon are extracted from the top and from the bottom of the distillation column, respectively.
One of the experiments that study dark matter at the INFN Gran Sasso National Laboratory is XENON1T, which uses large quantities of xenon as material in which to detect dark matter (see Dark Matter). However, this element naturally contains traces of radon and krypton, two radioactive elements that interfere with the measurements. In fact, their natural decay towards stable elements causes the production of particles that can mask the sought-after dark matter interactions. To eliminate these two contaminants a distillation column is used: an apparatus in which their different vapour pressure is exploited. This is a physical property characteristic of each element, which indicates its volatility, i.e. a measure of the probability of the element passing from the liquid phase to the gas phase, at a given temperature and pressure. In a closed container with an element in the liquid and gaseous phase in a state of thermodynamic equilibrium, at a certain interval of time there will be an equal amount of molecules passing from the liquid to the gaseous state and the other way around. If there is a mixture of several elements with different volatility, it is possible to use the phase change to separate them. This is the principle of any distillation process.
Krypton has a volatility about ten times greater than that of xenon, so when the liquid and gas phases of xenon are in equilibrium, the krypton will on average be ten times more likely to evaporate. After a certain time most of the krypton will have transferred to the gaseous phase of the mixture, being less present in the liquid. Within the distillation column, several cycles of these phase changes are induced to gradually reduce the concentration of krypton to the desired levels (Fig. 1). Xenon is pumped into the column at a temperature and pressure at which part of it goes into a gaseous flow directed upwards and part of it flows downwards in the liquid phase. At the bottom of the column there is an 8-liter tank in which the liquid is collected and which is heated to keep the liquid boiling; at the top of the column there is a condenser. By properly adjusting the temperature and pressure inside the column, the heater and condenser ensure that there is a continuous flow of gas directed upwards and liquid directed downwards. Starting from the bottom, the mixture of xenon and krypton evaporates and the resulting gas condenses on a special plate above (Fig. 1). Since krypton evaporates more easily than xenon, the gas that arrives on the plate, and which condenses here, will proportionally contain more krypton than the liquid in the starting tank. Repeating this evaporation and condensation procedure about ten times, the gas reaches the top of the column with a high concentration of krypton. Here a part of this gas is expelled and a part ends up in the condenser which turns it back into liquid, allowing it to descend towards the tank at the bottom, where a new purification cycle will then start again. After completing various krypton reduction cycles, the purified xenon is collected at the bottom of the tank and pumped towards the detector. With this procedure it is possible to decrease the presence of krypton by about 500 thousand times, reaching the desired purity.
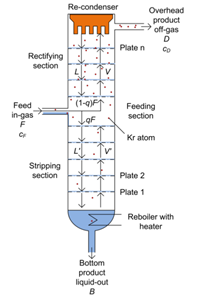
Fig. 1. Scheme of a distillation column to separate the krypton from xenon. The xenon to be purified (Feed in-gas) enters the column from the left, at half height; the liquid phase is deposited at the bottom, where a heater is placed. The xenon containing krypton evaporates and condenses on the first plate (Plate 1), then on the second plate and so on, up to plate n (Plate n). At each step the concentration of krypton increases. At the top of the column, part of the krypton-rich gas is expelled (in the picture on the right, Overhead product off-gas) and part is transformed by the condenser (Re-condenser) into liquid, which goes back down and is reintroduced into the cycle.
The amount of radon present in xenon is reduced by using the same chemical- physical principle used for krypton. In this case, the xenon has a higher volatility than radon (about ten times). The procedure is therefore the same, but at each stage of evaporation and condensation the xenon concentrates in the upper part of the column, in gaseous phase, while the radon evaporates less and remains more concentrated in the liquid phase. Therefore, at the end of a cycle the xenon with a high concentration of radon is collected in the liquid phase at the bottom, while the purified xenon gas is released from a tube near the condenser, at the top of the column (Fig. 2). In this way there is no loss of xenon and the concentration of radon decreases by about 20%.
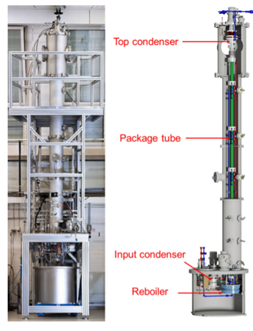
Fig. 2. (Left) Photograph of the distillation column and (right) a scheme of the column. From top to bottom one can see the Top Condenser, the Package Tube where xenon passes several times, the Input Condenser through which the xenon enters, and the Reboiler at the very bottom. The total height of the column is 5.5 m.

Discover the experiments and help the alien to get back home!
Play now